Abstract
Introduction:
Acute Graft versus Host Disease (aGvHD) occurs in 20-50% of pediatric patients who undergo allogeneic stem cell transplantation. Despite advances in HLA-typing methods and post-transplant immune suppression, it remains a significant cause of mortality and morbidity. Conditioning regimens include alkylating agents that exert their effects through their ability to directly or indirectly damage DNA. Unfortunately normal cells are also damaged due to their rapid proliferation cycles, increasing the probability of treatment-related toxicities. The most vulnerable targets among these damaged tissues are the skin, the intestinal epithelium, and the liver: In this study, our aim was to find biomarkers for aGvHD by focussing on how inter-individual differences in DNA repair mechanisms due to genetic variants in genes encoding DNA repair proteins might affect toxicity.
Methods:
The study included 115 children that had undergone allo-HSCT at four different centers. All patients received a Busulfan(Bu)/Cyclophosphamide(Cy) conditioning regimen. Pharmacokinetic-guided dose adjustment was performed for Bu to obtain a concentration at the steady state (Css) between 600 and 900 ng/ml. Patients received 16 doses of Bu followed by, i.v. Cy (200mg/kg total dose, 80% of patients) or CyVP16 (120mg/kg total dose). Cyclosporine was given as GvHD prophylaxis, and Methotrexate (MTX) or steroids were added to bone marrow and cord blood transplantation, respectively. ATG was given to 75% of patients. HLA matching was as follows: MRD = 37%; MUD = 21%; MMRD = 4%; MMUD = 38%. Acute GvHD was diagnosed according to the 1994 consensus conference up to day 180 post HSCT. Peripheral blood was collected and the DNA was extracted prior to transplant. Fifty-one single nucleotide polymorphisms (SNPs) within seventeen DNA repair genes were chosen for investigation. Cumulative incidence analysis of aGvHD 2-4 was performed using Kaplan-Meier analysis and log-rank test. Multivariate Cox regression was performed to estimate the impact of genotype on clinical outcome in the presence of other covariates.
Results:
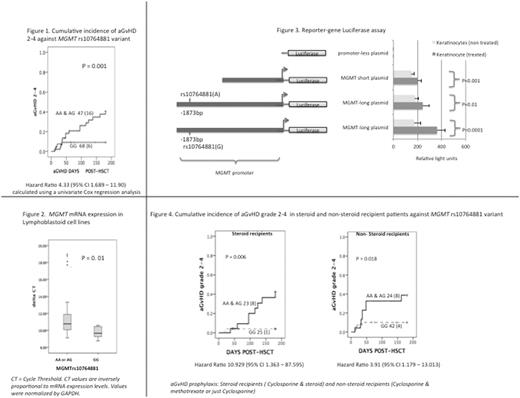
The most significant finding came from one SNP (rs10764881, G>A) located in the promoter of the MGMT gene. Patients with rs10764881 GG genotypes had a lower risk for aGvHD 2-4 incidence (Figure 1). This variant was not associated with any other treatment related toxicities nor relapses. Multivariate analysis including MGMT rs10764881 with known aGvHD risk covariates did not influence the model. In addition, from the expression analysis that we performed on 24 lymphoblastoid cell lines rs10764881 GG carriers showed 1.5 fold higher expression compared to AA or AG carriers (Figure 2). We were able to confirm experimentally with luciferase reporter constructs the impact of this variant on promoter function. Plasmids, which included the promoter sequence with rs10764881 demonstrated higher luciferase protein levels, compared to plasmids with a promoter sequence excluding rs10764881 (p= 0.01), suggesting the presence of an enhancement element close to the variant region (Figure 3). Electrophoretic mobility shift assays confirmed the presence of a Glucocorticoid Receptor Element (GRE) near to this variant. To understand whether the enhancing effects are related to corticosteroids, keratinocytes transfected with the gene reporter plasmids were stimulated with dexamethasone and luciferase expression was examined as previously. Exposing the cells with dexamethasone significantly increased protein expression of luciferase in both plasmids compared to their non-treated plasmid construct. The highest response was seen from the plasmid containing variant rs10764881 G (Figure 3). However, clinically we did not find clear differences between steroid and non-steroids recipients against rs10764881 with regards to aGvHD vulnerability (Figure 4).
Conclusions:
We hypothesize that the reason why variant rs10764881 GG might have a protective effect against aGvHD is due to its higher expression levels, resulting in more efficient DNA repair, in turn diminishing the immune response, reducing inflammation and hence causing less aGvHD. Thus aGvHD rs10764881 GG could potentially be a biomarker for aGvHD protection. The effects of steroids on MGMT expression needs to be addressed in future studies.
Bader: Novartis, Medac, Amgen, Riemser, Neovii: Consultancy, Honoraria, Research Funding. Bittencourt: Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Travel Grant; Amgen Inc.: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal